Guess what it was used for in the land of Upper and Lower Nile dwellers
Description
[edit]
The pyramid superstructure seem to have never been started, and the only information that can be determined from it is that the pyramid was planned larger than its southern neighbor, which has a side length of 52.5 m (172 ft).

The hypogeum is similar to the one of the southern pyramid but much more tortuous, changing direction six times. The entrance is on the north side. From there, a staircase leads down to a square chamber and then to another staircase and to the first quartzite blocking. After that, two other chambers are connected by a passage with a second, still unsealed blocking. After the third chamber, a stairway and then a corridor leads to the antechamber just prior to the large burial chamber: this room, partially covered by an inverted V-shaped ceiling, is entirely filled by a huge sarcophagus-vault, which was carved from a single block of quartzite. The never-used sarcophagus lid, a 42-ton quartzite slab, still awaits to be fitted in the chamber. All exposed quartzite, which was built in the pyramid, had been painted with red paint and sometimes also decorated with vertical black stripes. The function of a large room behind the burial chamber remain unknown.[4][5]
From the pyramid complex, the valley temple, the funerary temple and the enclosure walls had apparently left no traces. Only a large portion of the causeway has been discovered, as well as another blocking stone, likely abandoned due to a change of the pyramid's design.
Hints for free
Snow fences work by causing turbulence in the wind, such that it drops much of its snow load on the lee side of the fence. Thus, snow fences actually cause snow drifts, rather than preventing them. The fences are placed so as to cause snow to drift where it is beneficial, or not harmful so that the snow does not drift onto undesired areas such as roads or among buildings.
Nitrogen
 Liquid nitrogen (N2 at below −196 °C) | |||||||||||||||||||||||||||||||||
| Nitrogen | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allotropes | see § Allotropes | ||||||||||||||||||||||||||||||||
| Appearance | colorless gas, liquid or solid | ||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(N) | |||||||||||||||||||||||||||||||||
| Nitrogen in the periodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number (Z) | 7 | ||||||||||||||||||||||||||||||||
| Group | group 15 (pnictogens) | ||||||||||||||||||||||||||||||||
| Period | period 2 | ||||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [He] 2s2 2p3 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 5 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| Phase at STP | gas | ||||||||||||||||||||||||||||||||
| Melting point | (N2) 63.23[3] K (−209.86[3] °C, −345.75[3] °F) | ||||||||||||||||||||||||||||||||
| Boiling point | (N2) 77.355 K (−195.795 °C, −320.431 °F) | ||||||||||||||||||||||||||||||||
| Density (at STP) | 1.2506 g/L[4] at 0 °C, 1013 mbar | ||||||||||||||||||||||||||||||||
| when liquid (at b.p.) | 0.808 g/cm3 | ||||||||||||||||||||||||||||||||
| Triple point | 63.151 K, 12.52 kPa | ||||||||||||||||||||||||||||||||
| Critical point | 126.21 K, 3.39 MPa | ||||||||||||||||||||||||||||||||
| Heat of fusion | (N2) 0.72 kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporisation | (N2) 5.57 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | (N2) 29.124 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapour pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | −3, −2, −1, 0,[5] +1, +2, +3, +4, +5 (a strongly acidic oxide) | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 3.04 | ||||||||||||||||||||||||||||||||
| Ionisation energies |
| ||||||||||||||||||||||||||||||||
| Covalent radius | 71±1 pm | ||||||||||||||||||||||||||||||||
| Van der Waals radius | 155 pm | ||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal (hP4) | ||||||||||||||||||||||||||||||||
| Lattice constants | a = 411.6 pm c = 673.4 pm (at t.p.)[6] | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 25.83×10−3 W/(m⋅K) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||||||||||||||
| Speed of sound | 353 m/s (gas, at 27 °C) | ||||||||||||||||||||||||||||||||
| CAS Number | 17778-88-0 7727-37-9 (N2) | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Discovery | Daniel Rutherford (1772) | ||||||||||||||||||||||||||||||||
| Named by | Jean-Antoine Chaptal (1790) | ||||||||||||||||||||||||||||||||
| Isotopes of nitrogen | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Nitrogen is a chemical element; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name nitrogène was suggested by French chemist Jean-Antoine-Claude Chaptal in 1790 when it was found that nitrogen was present in nitric acid and nitrates. Antoine Lavoisier suggested instead the name azote, from the Ancient Greek: ἀζωτικός "no life", as it is an asphyxiant gas; this name is used in a number of languages, and appears in the English names of some nitrogen compounds such as hydrazine, azides and azo compounds.
Elemental nitrogen is usually produced from air by pressure swing adsorption technology. About 2/3 of commercially produced elemental nitrogen is used as an inert (oxygen-free) gas for commercial uses such as food packaging, and much of the rest is used as liquid nitrogen in cryogenic applications. Many industrially important compounds, such as ammonia, nitric acid, organic nitrates (propellants and explosives), and cyanides, contain nitrogen. The extremely strong triple bond in elemental nitrogen (N≡N), the second strongest bond in any diatomic molecule after carbon monoxide (CO),[7] dominates nitrogen chemistry. This causes difficulty for both organisms and industry in converting N2 into useful compounds, but at the same time it means that burning, exploding, or decomposing nitrogen compounds to form nitrogen gas releases large amounts of often useful energy. Synthetically produced ammonia and nitrates are key industrial fertilisers, and fertiliser nitrates are key pollutants in the eutrophication of water systems. Apart from its use in fertilisers and energy stores, nitrogen is a constituent of organic compounds as diverse as aramids used in high-strength fabric and cyanoacrylate used in superglue.
Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Nitrogen is a constituent of every major pharmacological drug class, including antibiotics. Many drugs are mimics or prodrugs of natural nitrogen-containing signal molecules: for example, the organic nitrates nitroglycerin and nitroprusside control blood pressure by metabolizing into nitric oxide. Many notable nitrogen-containing drugs, such as the natural caffeine and morphine or the synthetic amphetamines, act on receptors of animal neurotransmitters.
Snow aka water at a
turbulent state and a
solid ic crystal stage
as it falls from the pool
of water that birthed each drop
but not its entire content
as the magic magnetic moment
perfected over in ch Σs of scraping
along the surface of the earth
collecting dirt water frogs
seeds
forming a pool often called a cloud
because look at that lake in the sky
would scare too many drivers and cause
more than the already absurd number of
automobile accidents that occur daily
The lake in the sky is magnetically aligned
with the surface of the earth below which
tends to follow the charge pattern
suggested by light
being more positively off at night out of the light
and more negatively on during the electron poke
where the sun reaches out to the earth with tree
finger tips
the pinky touches the western edge wherelast night is being greeted with the proper
tea position for any well bred pinky
The two middle fingers
find some raspy edge to get a little
fric t ion
going eastward where
the index finger is pointing to tomorrow
the day that began last yesterday
and never arrives as the
busy are too busy being
where now
know here
there is the rub where the hot hot
sun with a finger pretending to be a gun
spills the beans as the attractive day
pushed along by two to the left
likes the message in the massage and
is ready for bed two by to
then as the world turns
the 24000 mile arc
of now is tickled at
the edges into
a state of ticked pink
when
sooner and
later
where as the orb or b its itself
round at 1000 mph
the atmost 240,000 miles of
layered spheres arranged
magneto matic ally holding
releasing gathering up
reaping sowing
sweeping
formations which are glasses of water
waiting for a glass
to fill
a nor th west aligned
toroidal cylinder
crossing Sou th East laminate layers
Positively holding the water
like any young child must
wait
in
g
for the next stop
when suddenlYY
ZYZ
ZYz
YZYZ
xyxyxyxyxyxyxyxyxyxyxyxYZ
YZ
YZ
YZYZ
YZY
ZyZyzYzYZYZYZYzyZYZYZYZYZYZYZYZYZYZYZYZYZYZYZYZY
Electrons are ripped from balanced
orbits
creating
a Pool of
Negative charge
Masquerading a the waiting water
until Wala
the magnetic moment holding the
water in suspension
above the
Positive
ly beautiful
flowing green
pasture
below
The term molecular sieve refers to a particular property of these materials, i.e., the ability to selectively sort molecules based primarily on a size exclusion process. This is due to a very regular pore structure of molecular dimensions. The maximum size of the molecular or ionic species that can enter the pores of a zeolite is controlled by the dimensions of the channels. These are conventionally defined by the ring size of the aperture, where, for example, the term "eight-ring" refers to a closed-loop that is built from eight tetrahedrally coordinated silicon (or aluminium) atoms and eight oxygen atoms. These rings are not always perfectly symmetrical due to a variety of causes, including strain induced by the bonding between units that are needed to produce the overall structure or coordination of some of the oxygen atoms of the rings to cations within the structure. Therefore, the pores in many zeolites are not cylindrical.
pumped up after a day of absorbing
Pressure swing adsorption (PSA) is a technique used to separate some gas species from a mixture of gases (typically air) under pressure according to the species' molecular characteristics and affinity for an adsorbent material. It operates at near-ambient temperature and significantly differs from the cryogenic distillation commonly used to separate gases. Selective adsorbent materials (e.g., zeolites, (aka molecular sieves), activated carbon, etc.) are used as trapping material, preferentially adsorbing the target gas species at high pressure. The process then swings to low pressure to desorb the adsorbed gas.
Process[edit]

| I | compressed air input | A | adsorption | |
|---|---|---|---|---|
| O | oxygen output | D | desorption | |
| E | exhaust |
The pressure swing adsorption (PSA) process is based on the phenomenon that under high pressure, gases tend to be trapped onto solid surfaces, i.e. to be "adsorbed". The higher the pressure, the more gas is adsorbed. When the pressure is dropped, the gas is released, or desorbed. PSA can be used to separate gases in a mixture because different gases are adsorbed onto a given solid surface more or less strongly. For example, if a gas mixture such as air is passed under pressure through a vessel containing an adsorbent bed of zeolite that attracts nitrogen more strongly than oxygen, a fraction of nitrogen will stay in the bed, and the gas exiting the vessel will be richer in oxygen than the mixture entering. When the bed reaches the limit of its capacity to adsorb nitrogen, it can be regenerated by decreasing the pressure, thus releasing the adsorbed nitrogen. It is then ready for another cycle of producing oxygen-enriched air.
Using two adsorbent vessels allows for near-continuous production of the target gas. It also allows a pressure equalisation, where the gas leaving the vessel being depressurised is used to partially pressurise the second vessel. This results in significant energy savings, and is a common industrial practice.
blue at the wavelength 432
with room for dessert as
Last call for
the day is
tipped by
a bell
in
ΘηΣ
Δ
ΙΓΕΚ
Τ
ΙΟΝ
most casually known as down
leaving the snow where the farmer will
use the water when the water
is in the
coherent 4 degree
state of states
loose limber looking for love
Nirtogen has a frozen crystal to boiling cloud range of 15 degrees from 3
Nitrogen compounds have a very long history, ammonium chloride having been known to Herodotus. They were well-known by the Middle Ages. Alchemists knew nitric acid as aqua fortis (strong water), as well as other nitrogen compounds such as ammonium salts and nitrate salts. The mixture of nitric and hydrochloric acids was known as aqua regia (royal water), celebrated for its ability to dissolve gold, the king of metals.[8]
and Joseph Priestley,[14] who referred to it as burnt air or phlogisticated air. French chemist Antoine Lavoisier referred to nitrogen gas as "mephitic air" or azote, from the Greek word άζωτικός (azotikos), "no life", due to it being asphyxiant.[15][16] In an atmosphere of pure nitrogen, animals died and flames were extinguished. Though Lavoisier's name was not accepted in English since it was pointed out that all gases but oxygen are either asphyxiant or outright toxic, it is used in many languages (French, Italian, Portuguese, Polish, Russian, Albanian, Turkish, etc.; the German Stickstoff similarly refers to the same characteristic, viz. ersticken "to choke or suffocate") and still remains in English in the common names of many nitrogen compounds, such as hydrazine and compounds of the azide ion. Finally, it led to the name "pnictogens" for the group headed by nitrogen, from the Greek πνίγειν "to choke".[8]
Zeolites are widely used as ion-exchange beds in domestic and commercial water purification, softening, and other applications.
Evidence for the oldest known zeolite water purification filtration system occurs in the undisturbed sediments of the Corriental reservoir at the Maya city of Tikal, in northern Guatemala.[27]
Earlier, polyphosphates were used to soften hard water. The polyphosphates forms complex with metal ions like Ca2+ and Mg2+ to bind them up so that they could not interfere in cleaning process. However, when this phosphate rich water goes in main stream water, it results in eutrophication of water bodies and hence use of polyphosphate was replaced with use of a synthetic zeolite.
The largest single use for zeolite is the global laundry detergent market. Zeolites are used in laundry detergent as water softeners, removing Ca2+ and Mg2+ ions which would otherwise precipitate from the solution. The ions are retained by the zeolites which releases Na+ ions into the solution, allowing the laundry detergent to be effective in areas with hard water.[28]
Forms the ball that is pushed
by the kepher at sunrise
towards the sun
| Ra | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
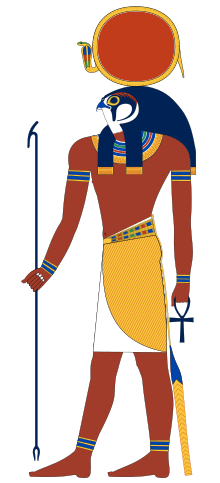 | |||||||||||
| Name in hieroglyphs | or or | ||||||||||
| Major cult center | Heliopolis but was worshipped everywhere in Ancient Egypt. | ||||||||||
| Symbol | Sun Disk | ||||||||||
| Genealogy | |||||||||||
| Parents | None (most accounts) Khnum and Neith (alternative sources) Hathor (In the cycle of rebirth) Mehet-Weret (some accounts) | ||||||||||
| Siblings | Apep, Sobek and Serket (as son of Khnum and Neith) | ||||||||||
| Consort | Hathor, Sekhmet, Bastet, Satet (in some myths) | ||||||||||
| Offspring | Shu, Tefnut, Hathor, Sekhmet, Mafdet, Bastet, Satet, Anhur, Ma'at, Mut | ||||||||||
| Equivalents | |||||||||||
| Greek equivalent | Helios[1] | ||||||||||
the snake lives
at the horizon
coming to get itselffrom the other end
In Greek mythology, Aether, Æther, Aither, or Ether (/ˈiːθər/; Ancient Greek: Αἰθήρ (Brightness)[1] pronounced [ai̯tʰɛ̌ːr]) is the personification of the bright upper sky. According to Hesiod, he was the son of Erebus (Darkness) and Nyx (Night), and the brother of Hemera (Day).[2] In Orphic cosmogony Aether was the offspring of Chronos (Time), and the brother of Chaos and Erebus.[3]














.png)





Comments
Post a Comment